
Metals like copper and silver occur in a free state and the combined state in the Earth’s crust.
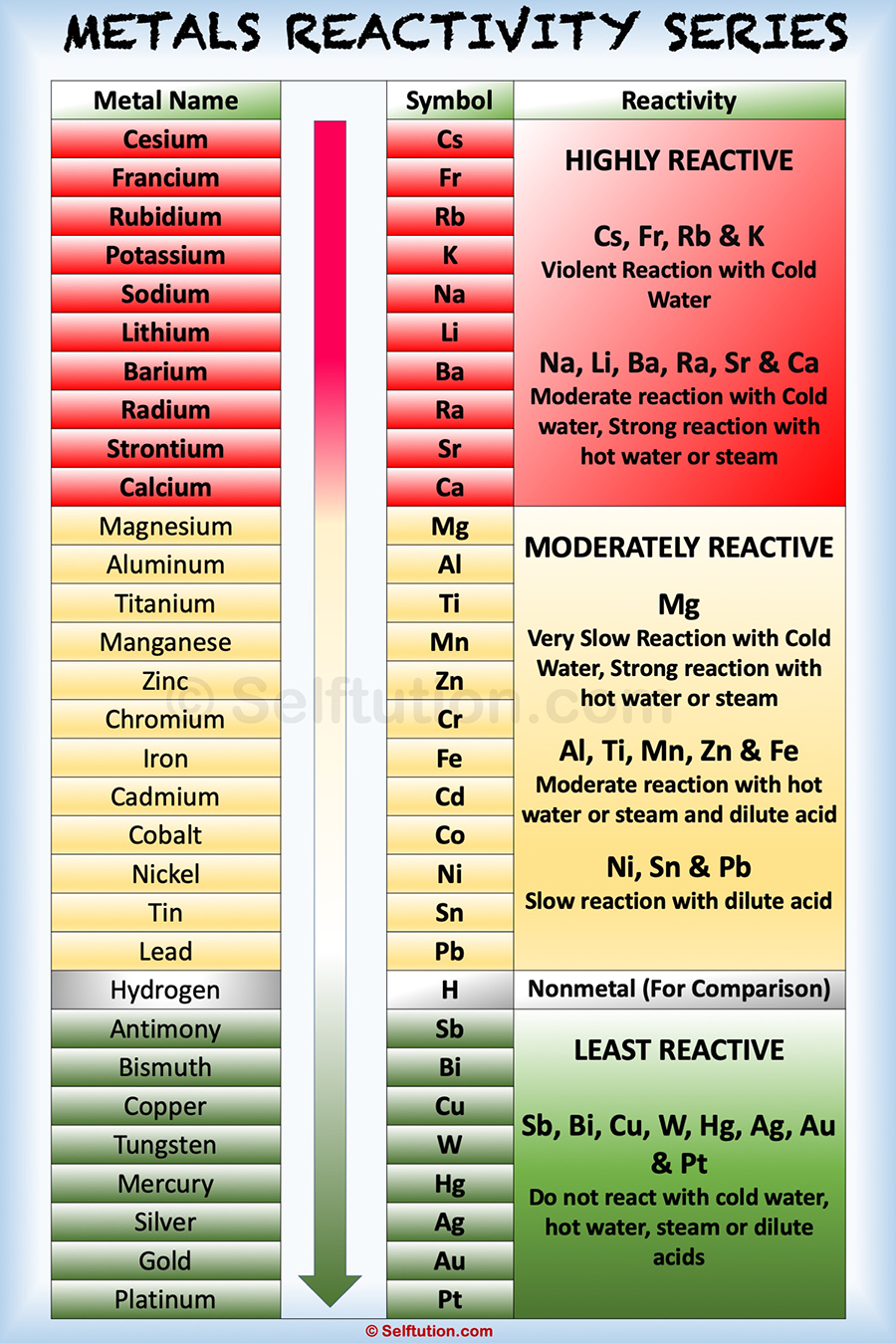
REACTIVITY OF METALS SERIES
The metals lying low in the reactivity series like gold and platinum occur in a free state. A metal may occur in its native state (free state) or combined state depending upon its position in the reactivity series of metals.
Seawater also contains some metal salts in an insoluble form. The primary source of metals is the Earth’s crust. The desired metal complex, as well as impurities and earthy things known as Gangue, make up ore. We can utilise the minerals in the earth because of the extraction of metals from ores! The ores are not the same as the finished metals seen in structures and bridges. Metal ores can be found in variable quantities in the earth’s crust. Mining is the process of recovering metal ores from deep down. Although an element may combine with a number of other elements to form a variety of minerals, only a handful of them is viable sources of that metal. The majority of elements, particularly metals, are found in combination with other elements and are referred to as minerals.

Metallurgy is the branch of science that deals with extracting metals from ores that are present naturally in the environment.

Only a few metals, such as noble metals such as gold, silver, and platinum, are found in their natural metallic forms. Extraction of Metals: The purpose of Isolation of Elements in Chemistry Class 12 is to teach students about different methods of extracting metals from ores.


 0 kommentar(er)
0 kommentar(er)
